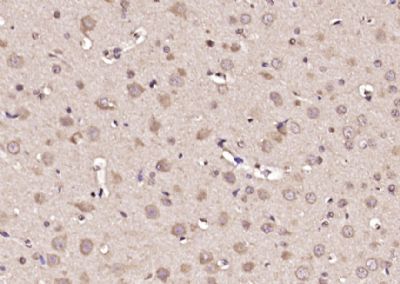
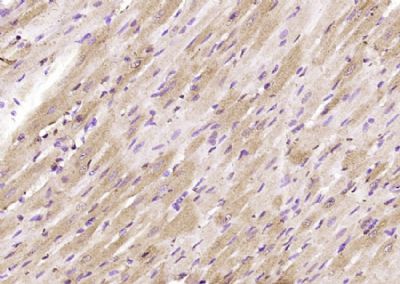
Glypican 1 Polyclonal Antibody
Purified Rabbit Polyclonal Antibody (Pab)
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| IHC-P, IHC-F, IF, ICC, E |
|---|---|
| Primary Accession | Q9QZF2 |
| Reactivity | Rat, Dog, Bovine |
| Host | Rabbit |
| Clonality | Polyclonal |
| Calculated MW | 62 KDa |
| Physical State | Liquid |
| Immunogen | KLH conjugated synthetic peptide derived from mouse Glypican 1 |
| Epitope Specificity | 251-350/557 |
| Isotype | IgG |
| Purity | affinity purified by Protein A |
| Buffer | 0.01M TBS (pH7.4) with 1% BSA, 0.02% Proclin300 and 50% Glycerol. |
| SUBCELLULAR LOCATION | Cell membrane; Lipid-anchor, GPI-anchor; Extracellular side. Endosome. Note=S-nitrosylated form recycled in endosomes. Localizes to CAV1-containing vesicles close to the cell surface. Cleavage of heparan sulfate side chains takes place mainly in late endosomes. Associates with both forms of PRNP in lipid rafts. Colocalizes with APP in perinuclear compartments and with CP in intracellular compartments. Associates with fibrillar APP Abeta peptides in lipid rafts in Alzheimer disease brains. Secreted glypican-1: Secreted, extracellular space. |
| SIMILARITY | Belongs to the glypican family. |
| Post-translational modifications | S-nitrosylated in a Cu(2+)-dependent manner. Nitric acid (NO) is released from the nitrosylated cysteines by ascorbate or by some other reducing agent, in a Cu(2+) or Zn(2+) dependent manner. This free nitric oxide is then capable of cleaving the heparan sulfate side chains. N- and O-glycosylated. N-glycosylation is mainly of the complex type containing sialic acid. O-glycosylated with heparin sulfate. The heparan sulfate chains can be cleaved either by the action of heparanase or, degraded by a daaminative process that uses nitric oxide (NO) released from the S-nitrosylated cysteines. This process is triggered by ascorbate, or by some other reducing agent, in a Cu(2+)- or Zn(2+) dependent manner. Cu(2+) ions are provided by ceruloproteins such as APP, PRNP or CP which associate with GCP1 in intracellular compartments or lipid rafts. This cell-associated glypican is further processed to give rise to a medium-released species. |
| DISEASE | Note=Associates (via the heparan sulfate side chains) with fibrillar APP-beta amyloid peptides in primitive and classic amyloid plaques and may be involved in the deposition of these senile plaques in the Alzheimer disease (AD) brain. Note=Misprocessing of GPC1 is found in fibroblasts of patients with Niemann-Pick Type C1 disease. This is due to the defective deaminative degradation of heparan sulfate chains. |
| Important Note | This product as supplied is intended for research use only, not for use in human, therapeutic or diagnostic applications. |
| Background Descriptions | Cell surface heparan sulfate proteoglycans are composed of a membrane-associated protein core substituted with a variable number of heparan sulfate chains. Members of the glypican-related integral membrane proteoglycan family (GRIPS) contain a core protein anchored to the cytoplasmic membrane via a glycosyl phosphatidylinositol linkage. These proteins may play a role in the control of cell division and growth regulation. [provided by RefSeq, Jul 2008] |
| Gene ID | 14733 |
|---|---|
| Other Names | Glypican-1, Secreted glypican-1, Gpc1 |
| Dilution | IHC-P=1:100-500,IHC-F=1:100-500,ICC=1:100-500,IF=1:100-500,Flow-Cyt=2ug/Test,ELISA=1:5000-10000 |
| Format | 0.01M TBS(pH7.4), 0.09% (W/V) sodium azide and 50% Glyce |
| Storage | Store at -20 ℃ for one year. Avoid repeated freeze/thaw cycles. When reconstituted in sterile pH 7.4 0.01M PBS or diluent of antibody the antibody is stable for at least two weeks at 2-4 ℃. |
| Name | Gpc1 |
|---|---|
| Function | Cell surface proteoglycan that bears heparan sulfate. Binds, via the heparan sulfate side chains, alpha-4 (V) collagen and participates in Schwann cell myelination (By similarity). May act as a catalyst in increasing the rate of conversion of prion protein PRPN(C) to PRNP(Sc) via associating (via the heparan sulfate side chains) with both forms of PRPN, targeting them to lipid rafts and facilitating their interaction. Required for proper skeletal muscle differentiation by sequestering FGF2 in lipid rafts preventing its binding to receptors (FGFRs) and inhibiting the FGF-mediated signaling. Binds Cu(2+) or Zn(2+) ions. |
| Cellular Location | Cell membrane; Lipid-anchor, GPI-anchor; Extracellular side. Endosome. Note=S-nitrosylated form recycled in endosomes. Localizes to CAV1-containing vesicles close to the cell surface. Cleavage of heparan sulfate side chains takes place mainly in late endosomes. Associates with both forms of PRNP in lipid rafts Colocalizes with APP in perinuclear compartments and with CP in intracellular compartments. Associates with fibrillar APP amyloid-beta peptides in lipid rafts in Alzheimer disease brains |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.