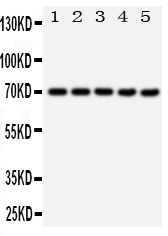
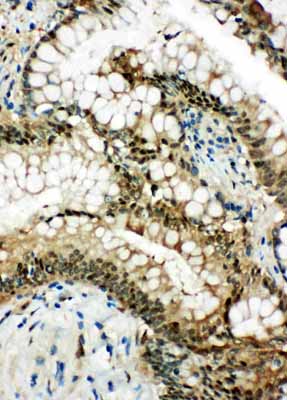
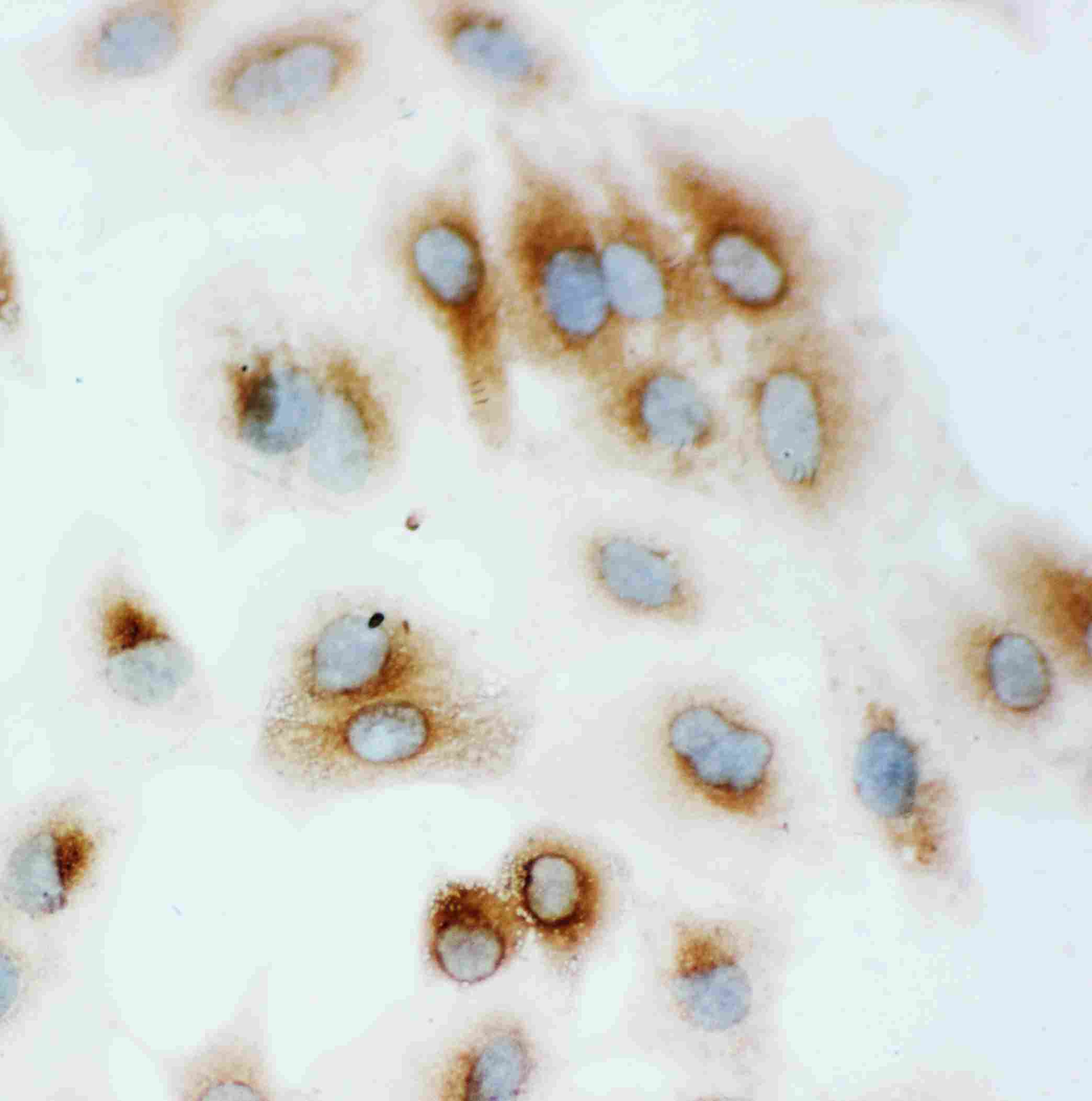
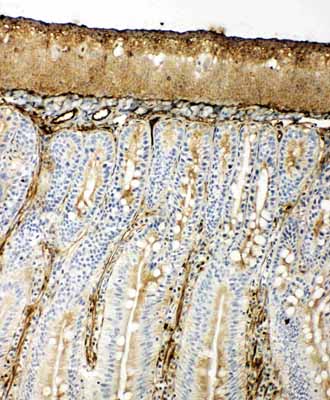
Anti-Hsp70 Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, IHC-P, IHC-F, ICC |
|---|---|
| Primary Accession | P0DMV8 |
| Host | Rabbit |
| Reactivity | Human, Rat |
| Clonality | Polyclonal |
| Format | Lyophilized |
| Description | Rabbit IgG polyclonal antibody for Heat shock 70 kDa protein 1A/1B(HSPA1A/HSPA1B) detection. Tested with WB, IHC-P, IHC-F, ICC in Human;Rat. |
| Reconstitution | Add 0.2ml of distilled water will yield a concentration of 500ug/ml. |
| Gene ID | 3303;3304 |
|---|---|
| Other Names | Heat shock 70 kDa protein 1A {ECO:0000312|HGNC:HGNC:5232}, Heat shock 70 kDa protein 1, HSP70-1, HSP70.1, HSPA1A, HSPA1, HSX70 |
| Calculated MW | 70052 MW KDa |
| Application Details | Immunohistochemistry(Paraffin-embedded Section), 0.5-1 µg/ml, Human, By Heat Immunocytochemistry , 0.5-1 µg/ml, Human, - Immunohistochemistry(Frozen Section), 0.5-1 µg/ml, Rat, Human Western blot, 0.1-0.5 µg/ml, Human |
| Subcellular Localization | Cytoplasm . Localized in cytoplasmic mRNP granules containing untranslated mRNAs. |
| Tissue Specificity | HSPA1B is testis-specific. |
| Protein Name | Heat shock 70 kDa protein 1A/1B |
| Contents | Each vial contains 5mg BSA, 0.9mg NaCl, 0.2mg Na2HPO4, 0.05mg Thimerosal, 0.05mg NaN3. |
| Immunogen | A synthetic peptide corresponding to a sequence at the C-terminus of human Hsp70(577-596aa VISWLDANTLAEKDEFEHKR), different from the related mouse sequence by four amino acids and from the related rat sequence by three amino acids. |
| Purification | Immunogen affinity purified. |
| Cross Reactivity | No cross reactivity with other proteins |
| Storage | At -20˚C for one year. After r˚Constitution, at 4˚C for one month. It˚Can also be aliquotted and stored frozen at -20˚C for a longer time.Avoid repeated freezing and thawing. |
| Sequence Similarities | Belongs to the heat shock protein 70 family. |
| Name | HSPA1A |
|---|---|
| Synonyms | HSP72 {ECO:0000303|PubMed:24318877}, HSP |
| Function | Molecular chaperone implicated in a wide variety of cellular processes, including protection of the proteome from stress, folding and transport of newly synthesized polypeptides, activation of proteolysis of misfolded proteins and the formation and dissociation of protein complexes. Plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, the re-folding of misfolded proteins and controlling the targeting of proteins for subsequent degradation. This is achieved through cycles of ATP binding, ATP hydrolysis and ADP release, mediated by co-chaperones. The co- chaperones have been shown to not only regulate different steps of the ATPase cycle, but they also have an individual specificity such that one co-chaperone may promote folding of a substrate while another may promote degradation. The affinity for polypeptides is regulated by its nucleotide bound state. In the ATP-bound form, it has a low affinity for substrate proteins. However, upon hydrolysis of the ATP to ADP, it undergoes a conformational change that increases its affinity for substrate proteins. It goes through repeated cycles of ATP hydrolysis and nucleotide exchange, which permits cycles of substrate binding and release. The co-chaperones are of three types: J-domain co-chaperones such as HSP40s (stimulate ATPase hydrolysis by HSP70), the nucleotide exchange factors (NEF) such as BAG1/2/3 (facilitate conversion of HSP70 from the ADP-bound to the ATP-bound state thereby promoting substrate release), and the TPR domain chaperones such as HOPX and STUB1 (PubMed:24012426, PubMed:24318877, PubMed:26865365). Maintains protein homeostasis during cellular stress through two opposing mechanisms: protein refolding and degradation. Its acetylation/deacetylation state determines whether it functions in protein refolding or protein degradation by controlling the competitive binding of co-chaperones HOPX and STUB1. During the early stress response, the acetylated form binds to HOPX which assists in chaperone-mediated protein refolding, thereafter, it is deacetylated and binds to ubiquitin ligase STUB1 that promotes ubiquitin-mediated protein degradation (PubMed:27708256). Regulates centrosome integrity during mitosis, and is required for the maintenance of a functional mitotic centrosome that supports the assembly of a bipolar mitotic spindle (PubMed:27137183). Enhances STUB1-mediated SMAD3 ubiquitination and degradation and facilitates STUB1-mediated inhibition of TGF-beta signaling (PubMed:24613385). Essential for STUB1-mediated ubiquitination and degradation of FOXP3 in regulatory T-cells (Treg) during inflammation (PubMed:23973223). Required as a co-chaperone for optimal STUB1/CHIP ubiquitination of NFATC3 (By similarity). Negatively regulates heat shock-induced HSF1 transcriptional activity during the attenuation and recovery phase period of the heat shock response (PubMed:9499401). Involved in the clearance of misfolded PRDM1/Blimp-1 proteins. Sequesters them in the cytoplasm and promotes their association with SYNV1/HRD1, leading to proteasomal degradation (PubMed:28842558). |
| Cellular Location | Cytoplasm. Nucleus. Cytoplasm, cytoskeleton, microtubule organizing center, centrosome. Secreted {ECO:0000250|UniProtKB:Q61696}. Note=Localized in cytoplasmic mRNP granules containing untranslated mRNAs |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
HSPA1(heat shock 70kDa protein 1A) also known as HSP70-1, HSPA1A, HSP70-1A, HSP72 or HSP70I, is a protein that in humans is encoded by the HSPA1A gene. This intronless gene encodes a 70kDa heat shock protein which is a member of the heat shock protein 70 family. The HSPA1A gene encodes a predicted 641-amino acid protein. The HSPA1 gene is mapped on 6p21.33. Shimizu et al.(1999) found that peripheral blood mononuclear cells of 18 major depression patients expressed a short HSPA1A transcript that utilized exon 1 rather than exon 2, which is found in the more common HSPA1A transcript. No protein was associated with expression of this short HSPA1A mRNA, possibly due to lack of a TATA box or loss of internal ribosome binding sites. Treatment with BGP-15, a pharmacologic inducer of Hsp72 that can protect against obesity-induced insulin resistance, improved muscular architecture, strength, and contractile function in severely affected diaphragm muscles in mdx dystrophic mice.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.











 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.