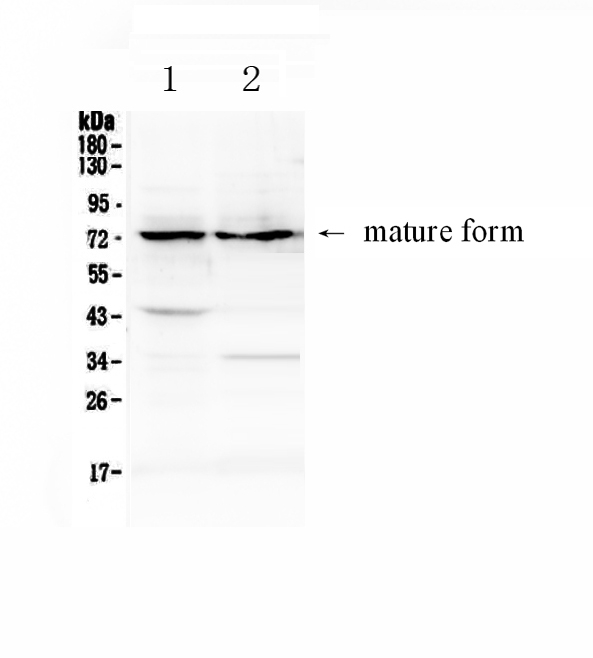
Anti-ADAM10 Picoband Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, E |
|---|---|
| Primary Accession | O14672 |
| Host | Rabbit |
| Reactivity | Human, Mouse, Rat |
| Clonality | Polyclonal |
| Format | Lyophilized |
| Description | Rabbit IgG polyclonal antibody for ADAM10 detection. Tested with WB, Direct ELISA in Human;Mouse;Rat. |
| Reconstitution | Add 0.2ml of distilled water will yield a concentration of 500ug/ml. |
| Gene ID | 102 |
|---|---|
| Other Names | Disintegrin and metalloproteinase domain-containing protein 10, ADAM 10, 3.4.24.81, CDw156, Kuzbanian protein homolog, Mammalian disintegrin-metalloprotease, CD156c, ADAM10, KUZ, MADM |
| Calculated MW | 84142 Da |
| Application Details | Western blot, 0.1-0.5 µg/ml Direct ELISA, 0.1-0.5 µg/ml |
| Subcellular Localization | Cell membrane. |
| Tissue Specificity | Expressed in spleen, lymph node, thymus, peripheral blood leukocyte, bone marrow, cartilage, chondrocytes and fetal liver. |
| Contents | Each vial contains 4mg Trehalose, 0.9mg NaCl, 0.2mg Na2HPO4, 0.05mg NaN3. |
| Immunogen | E. coli-derived human ADAM10 recombinant protein (Position: T214-D325). |
| Cross Reactivity | No cross reactivity with other proteins. |
| Storage | At -20˚C; for one year. After r˚Constitution, at 4˚C; for one month. It˚Can also be aliquotted and stored frozen at -20˚C; for a longer time. Avoid repeated freezing and thawing. |
| Name | ADAM10 (HGNC:188) |
|---|---|
| Synonyms | KUZ, MADM |
| Function | Transmembrane metalloprotease which mediates the ectodomain shedding of a myriad of transmembrane proteins, including adhesion proteins, growth factor precursors and cytokines being essential for development and tissue homeostasis (PubMed:11786905, PubMed:12475894, PubMed:20592283, PubMed:24990881, PubMed:26686862, PubMed:28600292, PubMed:31792032). Associates with six members of the tetraspanin superfamily TspanC8 which regulate its exit from the endoplasmic reticulum and its substrate selectivity (PubMed:26686862, PubMed:28600292, PubMed:31792032, PubMed:34739841, PubMed:37516108). Cleaves the membrane-bound precursor of TNF-alpha at '76-Ala-|-Val-77' to its mature soluble form. Responsible for the proteolytical release of soluble JAM3 from endothelial cells surface (PubMed:20592283). Responsible for the proteolytic release of several other cell-surface proteins, including heparin-binding epidermal growth-like factor, ephrin-A2, CD44, CDH2 and for constitutive and regulated alpha- secretase cleavage of amyloid precursor protein (APP) (PubMed:11786905, PubMed:26686862, PubMed:29224781, PubMed:34739841). Contributes to the normal cleavage of the cellular prion protein (PubMed:11477090). Involved in the cleavage of the adhesion molecule L1 at the cell surface and in released membrane vesicles, suggesting a vesicle-based protease activity (PubMed:12475894). Also controls the proteolytic processing of Notch and mediates lateral inhibition during neurogenesis (By similarity). Required for the development of type 1 transitional B cells into marginal zone B cells, probably by cleaving Notch (By similarity). Responsible for the FasL ectodomain shedding and for the generation of the remnant ADAM10-processed FasL (FasL APL) transmembrane form (PubMed:17557115). Also cleaves the ectodomain of the integral membrane proteins CORIN and ITM2B (PubMed:19114711, PubMed:21288900). Mediates the proteolytic cleavage of LAG3, leading to release the secreted form of LAG3 (By similarity). Mediates the proteolytic cleavage of IL6R and IL11RA, leading to the release of secreted forms of IL6R and IL11RA (PubMed:26876177). Enhances the cleavage of CHL1 by BACE1 (By similarity). Cleaves NRCAM (By similarity). Cleaves TREM2, resulting in shedding of the TREM2 ectodomain (PubMed:24990881). Involved in the development and maturation of glomerular and coronary vasculature (By similarity). During development of the cochlear organ of Corti, promotes pillar cell separation by forming a ternary complex with CADH1 and EPHA4 and cleaving CADH1 at adherens junctions (By similarity). May regulate the EFNA5-EPHA3 signaling (PubMed:16239146). Regulates leukocyte transmigration as a sheddase for the adherens junction protein VE- cadherin/CDH5 in endothelial cells (PubMed:28600292). |
| Cellular Location | Cell membrane; Single-pass type I membrane protein. Golgi apparatus membrane; Single-pass type I membrane protein. Cytoplasmic vesicle, clathrin-coated vesicle. Cell projection, axon {ECO:0000250|UniProtKB:O35598}. Cell projection, dendrite {ECO:0000250|UniProtKB:O35598}. Cell junction, adherens junction. Cytoplasm Note=Is localized in the plasma membrane but is also expressed in the Golgi apparatus and in clathrin-coated vesicles derived likely from the Golgi (PubMed:12475894). During long term depression, it is recruited to the cell membrane by DLG1 (PubMed:23676497). The immature form is mainly located near cytoplasmic fibrillar structures, while the mature form is predominantly located at zonula adherens and the cell membrane (PubMed:30463011). The localization and clustering of mature ADAM10 to zonula adherens is regulated by AFDN, TSPAN33, PLEKHA7 and PDZD11 (PubMed:30463011). |
| Tissue Location | Expressed in the brain (at protein level) (PubMed:23676497). Expressed in spleen, lymph node, thymus, peripheral blood leukocyte, bone marrow, cartilage, chondrocytes and fetal liver (PubMed:11511685, PubMed:9016778). |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
ADAM10, A Disintegrin and Metalloproteinase Domain 10, is also known as AD10. ADAM10 is a member of the ADAM family and members of this family are cell surface proteins with a unique structure possessing both potential adhesion and protease function. The ADAM10 gene is mapped to chromosome 15q21.3-q23. ADAM proteins contain an N-terminal signal sequence, followed by a prodomain, a metalloprotease-like domain, a disintegrin-like domain, a cysteine-rich region, an EGF -like repeat, a transmembrane domain, and a C-terminal cytoplasmic tail. Conversion of the membrane-bound precursor to a secreted mature protein is mediated by a protease termed TNFA convertase. ADAM10 possesses TNFA convertase activity.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.