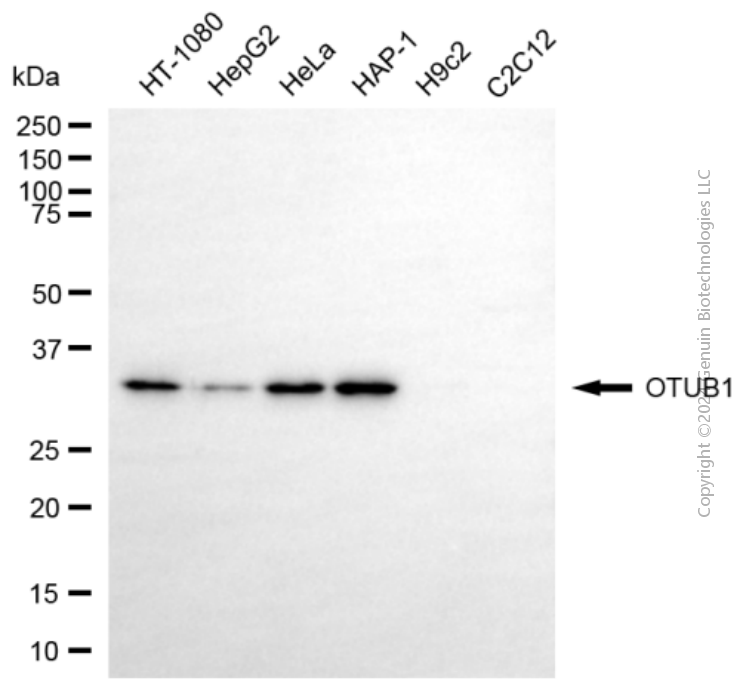
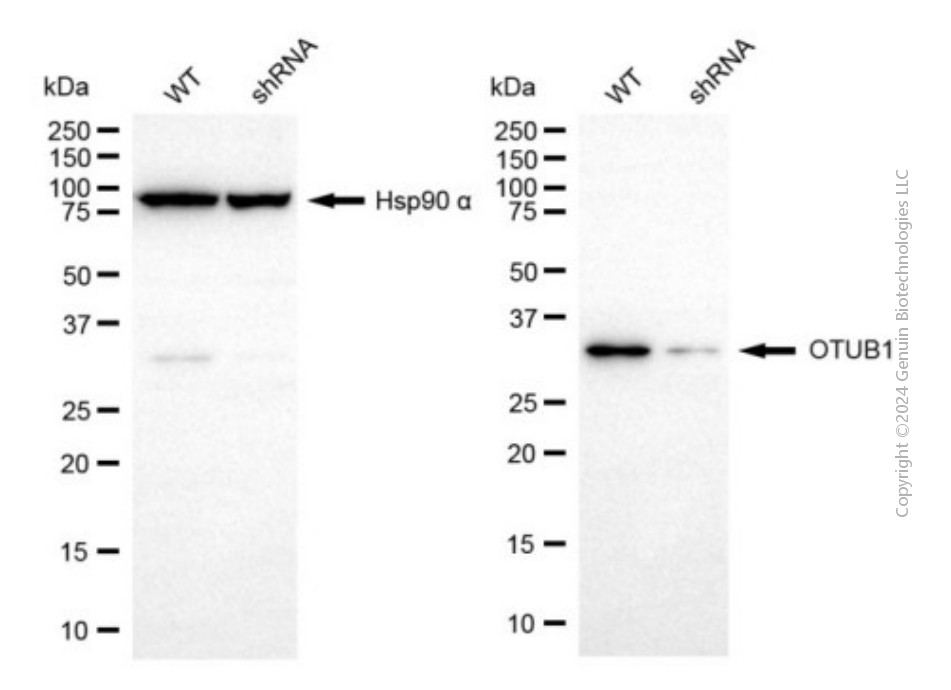
KD-Validated Anti-OTUB1 Mouse Monoclonal Antibody
Mouse monoclonal antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB |
|---|---|
| Primary Accession | Q96FW1 |
| Reactivity | Human |
| Clonality | Monoclonal |
| Isotype | Mouse IgG1 |
| Clone Names | 24GB6500 |
| Calculated MW | Predicted, 31 kDa, observed, 35 kDa |
| Gene Name | OTUB1 |
| Aliases | OTUB1; OTU Deubiquitinase, Ubiquitin Aldehyde Binding 1; OTU Domain-Containing Ubiquitin Aldehyde-Binding Protein 1; Ubiquitin-Specific-Processing Protease OTUB1; OTU Domain, Ubiquitin Aldehyde Binding 1; Deubiquitinating Enzyme OTUB1; Ubiquitin Thioesterase OTUB1; Otubain-1; FLJ20113; FLJ40710; OTB1; OTU1; Ubiquitin-Specific Protease Otubain 1; OTU-Domain Ubal-Binding 1; EC 3.4.19.12; EC 3.1.2; HSPC263; HOTU1 |
| Immunogen | Recombinant protein of human OTUB1 |
| Gene ID | 55611 |
|---|---|
| Other Names | Ubiquitin thioesterase OTUB1, 3.4.19.12, Deubiquitinating enzyme OTUB1, OTU domain-containing ubiquitin aldehyde-binding protein 1, Otubain-1, hOTU1, Ubiquitin-specific-processing protease OTUB1, OTUB1, OTB1, OTU1 |
| Name | OTUB1 |
|---|---|
| Synonyms | OTB1, OTU1 |
| Function | Hydrolase that can specifically remove 'Lys-48'-linked conjugated ubiquitin from proteins and plays an important regulatory role at the level of protein turnover by preventing degradation (PubMed:12401499, PubMed:12704427, PubMed:14661020, PubMed:23827681). Regulator of T-cell anergy, a phenomenon that occurs when T-cells are rendered unresponsive to antigen rechallenge and no longer respond to their cognate antigen (PubMed:14661020). Acts via its interaction with RNF128/GRAIL, a crucial inductor of CD4 T-cell anergy (PubMed:14661020). Isoform 1 destabilizes RNF128, leading to prevent anergy (PubMed:14661020). In contrast, isoform 2 stabilizes RNF128 and promotes anergy (PubMed:14661020). Surprisingly, it regulates RNF128- mediated ubiquitination, but does not deubiquitinate polyubiquitinated RNF128 (PubMed:14661020). Deubiquitinates estrogen receptor alpha (ESR1) (PubMed:19383985). Mediates deubiquitination of 'Lys-48'-linked polyubiquitin chains, but not 'Lys-63'-linked polyubiquitin chains (PubMed:18954305, PubMed:19211026, PubMed:23827681). Not able to cleave di-ubiquitin (PubMed:18954305, PubMed:23827681). Also capable of removing NEDD8 from NEDD8 conjugates, but with a much lower preference compared to 'Lys-48'-linked ubiquitin (PubMed:18954305, PubMed:23827681). |
| Cellular Location | Cytoplasm {ECO:0000250|UniProtKB:B2RYG6}. |
| Tissue Location | Isoform 1 is ubiquitous. Isoform 2 is expressed only in lymphoid tissues such as tonsils, lymph nodes and spleen, as well as peripheral blood mononuclear cells |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.














 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.