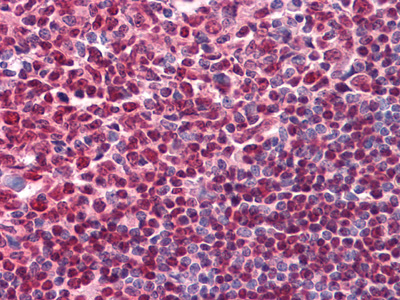
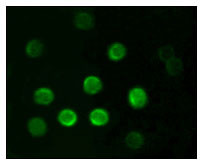
IFN-gamma Antibody
Purified Mouse Monoclonal Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, E |
|---|---|
| Primary Accession | P01579 |
| Reactivity | Human |
| Host | Mouse |
| Clonality | Monoclonal |
| Clone Names | 1B1A4; E8H1 |
| Isotype | IgG1 |
| Calculated MW | 19348 Da |
| Description | IFN-gamma (interferon, gamma) is an antiviral and antiparasitic agent produced by CD4+/CD8+ lymphocytes and natural killer cells that undergo activation by antigens, mitogens or alloantigens. It is a pleiotropic cytokine involved in the regulation of nearly all phases of immune and inflammatory responses, including the activation, growth and differentiation of T cell, B cells, macrophages, NK cells and other cell types such as endothelial cells and fibroblasts. The active form of IFN-G is a homodimer with each subunit containing six helices. The dimeric structure of human IFN-G is stabilized by non-covalent interactions through the interface of the helices. IFN-G translated precursor is 166 amino acids, including the 23 amino acid secretory sequence. It is upregulated by IL2, FGF basic, EGF and downregulated by vitamin D3 or DMN. Multiple forms exist due to variable glycosylation and under non-denaturing conditions due to dimers and tetramers. |
| Immunogen | Recombinant human IFN-gamma (BioSource company, Cat.No. PHC4033) |
| Formulation | Purified antibody in PBS containing 0.03% sodium azide. |
| Gene ID | 3458 |
|---|---|
| Other Names | Interferon gamma, IFN-gamma, Immune interferon, IFNG |
| Dilution | WB~~1/500 - 1/2000 E~~N/A |
| Storage | Maintain refrigerated at 2-8°C for up to 6 months. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. |
| Precautions | IFN-gamma Antibody is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | IFNG |
|---|---|
| Function | Type II interferon produced by immune cells such as T-cells and NK cells that plays crucial roles in antimicrobial, antiviral, and antitumor responses by activating effector immune cells and enhancing antigen presentation (PubMed:16914093, PubMed:8666937). Primarily signals through the JAK-STAT pathway after interaction with its receptor IFNGR1 to affect gene regulation (PubMed:8349687). Upon IFNG binding, IFNGR1 intracellular domain opens out to allow association of downstream signaling components JAK2, JAK1 and STAT1, leading to STAT1 activation, nuclear translocation and transcription of IFNG-regulated genes. Many of the induced genes are transcription factors such as IRF1 that are able to further drive regulation of a next wave of transcription (PubMed:16914093). Plays a role in class I antigen presentation pathway by inducing a replacement of catalytic proteasome subunits with immunoproteasome subunits (PubMed:8666937). In turn, increases the quantity, quality, and repertoire of peptides for class I MHC loading (PubMed:8163024). Increases the efficiency of peptide generation also by inducing the expression of activator PA28 that associates with the proteasome and alters its proteolytic cleavage preference (PubMed:11112687). Up-regulates as well MHC II complexes on the cell surface by promoting expression of several key molecules such as cathepsins B/CTSB, H/CTSH, and L/CTSL (PubMed:7729559). Participates in the regulation of hematopoietic stem cells during development and under homeostatic conditions by affecting their development, quiescence, and differentiation (By similarity). |
| Cellular Location | Secreted. |
| Tissue Location | Released primarily from activated T lymphocytes. |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
References
1. Dean GA. LaVoy A. Burkhard MJ. Vet Immunol Immunopathol. 2004,Jul, 100(1-2):49-59. 2. Arens R. Schepers K. Nolte MA. et al. J Exp Med. 2004,Jun 7, 199(11):1595-605. 3. Podhorecka M. Dmoszynska A. Rolinski J. Eur J Haematol. 2004,Jul, 73(1):29-35.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.