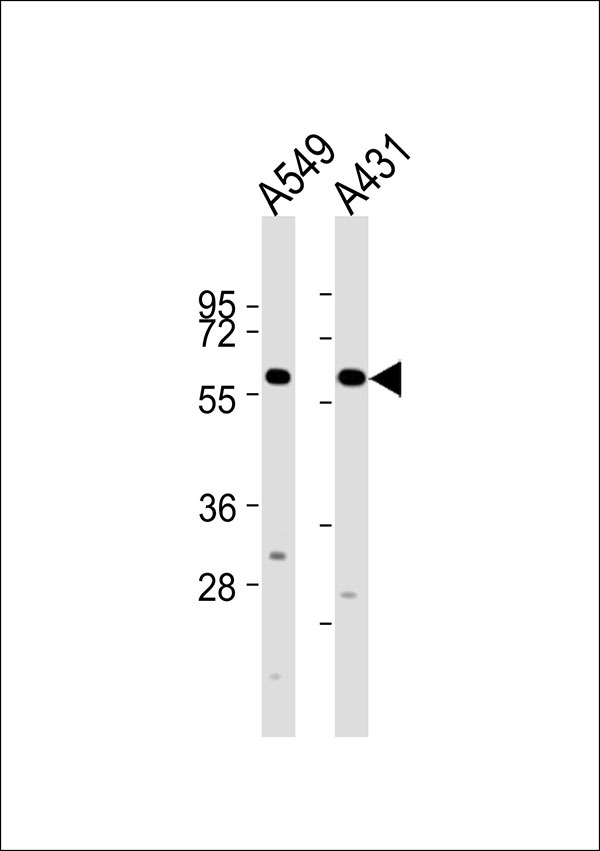
Cytochrome P450 2J2 Antibody
Purified Rabbit Polyclonal Antibody (Pab)
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB |
|---|---|
| Primary Accession | P51589 |
| Reactivity | Human, Mouse, Rat |
| Host | Rabbit |
| Clonality | Polyclonal |
| Calculated MW | 57 KDa |
| Antigen Region | 231 - 290 aa |
| Gene ID | 1573 |
|---|---|
| Other Names | Cytochrome P450 2J2, Arachidonic acid epoxygenase, CYPIIJ2, CYP2J2 |
| Target/Specificity | KLH-conjugated synthetic peptide encompassing a sequence within the center region of human Cytochrome P450 2J2. The exact sequence is proprietary. |
| Dilution | WB~~ 1:2000 |
| Format | 0.01M PBS, pH 7.2, 0.09% (W/V) Sodium azide, Glycerol 50% |
| Storage | Store at -20 °C.Stable for 12 months from date of receipt |
| Name | CYP2J2 {ECO:0000303|PubMed:19737933, ECO:0000312|HGNC:HGNC:2634} |
|---|---|
| Function | A cytochrome P450 monooxygenase involved in the metabolism of polyunsaturated fatty acids (PUFA) in the cardiovascular system (PubMed:19965576, PubMed:8631948). Mechanistically, uses molecular oxygen inserting one oxygen atom into a substrate, and reducing the second into a water molecule, with two electrons provided by NADPH via cytochrome P450 reductase (NADPH--hemoprotein reductase) (PubMed:19965576, PubMed:8631948). Catalyzes the epoxidation of double bonds of PUFA (PubMed:19965576, PubMed:8631948). Converts arachidonic acid to four regioisomeric epoxyeicosatrienoic acids (EpETrE), likely playing a major role in the epoxidation of endogenous cardiac arachidonic acid pools (PubMed:8631948). In endothelial cells, participates in eicosanoids metabolism by converting hydroperoxide species into hydroxy epoxy metabolites. In combination with 15- lipoxygenase metabolizes arachidonic acid and converts hydroperoxyicosatetraenoates (HpETEs) into hydroxy epoxy eicosatrienoates (HEETs), which are precursors of vasodilatory trihydroxyicosatrienoic acids (THETAs). This hydroperoxide isomerase activity is NADPH- and O2-independent (PubMed:19737933). Catalyzes the monooxygenation of a various xenobiotics, such as danazol, amiodarone, terfenadine, astemizole, thioridazine, tamoxifen, cyclosporin A and nabumetone (PubMed:19923256). Catalyzes hydroxylation of the anthelmintics albendazole and fenbendazole (PubMed:23959307). Catalyzes the sulfoxidation of fenbedazole (PubMed:19923256). |
| Cellular Location | Endoplasmic reticulum membrane; Peripheral membrane protein. Microsome membrane; Peripheral membrane protein |
| Tissue Location | Highly expressed in heart, present at lower levels in liver, kidney and skeletal muscle (at protein level) |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
This enzyme metabolizes arachidonic acid predominantly via a NADPH-dependent olefin epoxidation to all four regioisomeric cis-epoxyeicosatrienoic acids. One of the predominant enzymes responsible for the epoxidation of endogenous cardiac arachidonic acid pools.
References
Wu S.,et al.J. Biol. Chem. 271:3460-3468(1996).
Wu S.,et al.Submitted (JAN-2002) to the EMBL/GenBank/DDBJ databases.
King L.M.,et al.Mol. Pharmacol. 61:840-852(2002).
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.