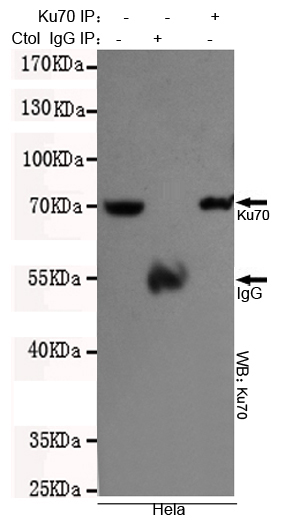
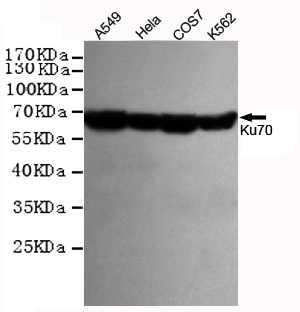
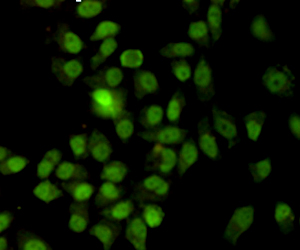
Ku70 Antibody
Purified Mouse Monoclonal Antibody (Mab)
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, ICC, IP |
|---|---|
| Primary Accession | P12956 |
| Reactivity | Human |
| Host | Mouse |
| Clonality | Monoclonal |
| Isotype | IgG1 |
| Calculated MW | 67 KDa |
| Gene ID | 2547 |
|---|---|
| Other Names | 5''-deoxyribose-5-phosphate lyase Ku70;5''-dRP lyase Ku70;70 kDa subunit of Ku antigen; ATP dependent DNA helicase 2 subunit 1;ATP dependent DNA helicase II 70 kDa subunit;ATP-dependent DNA helicase 2 subunit 1;ATP-dependent DNA helicase II 70 kDa subunit;CTC box binding factor 75 kDa subunit;CTC box-binding factor 75 kDa subunit;CTC75;CTCBF;CTCBF; DNA repair protein XRCC6;G22P1;Ku 70;Ku autoantigen 70kDa;Ku autoantigen p70 subunit;Ku autoantigen, 70kDa;Ku p70;Ku70;Ku70 DNA binding component of DNA-dependent proteinkinase complex (thyroid autoantigen 70 kDa;Kup70;Lupus Ku autoantigen protein p70;ML8;Thyroid autoantigen 70kD (Ku antigen);Thyroid autoantigen;Thyroid lupus autoantigen;Thyroid lupus autoantigen;Thyroid lupus autoantigen p70;Thyroid-lupus autoantigen;TLAA;TLAA;X ray repair complementing defective repair in Chinese hamster cells 6;X-ray repair complementing defective repair in Chinese hamster cells 6;X-ray repair cross-complementing protein 6;XRCC 6;XRCC6;XRCC6_HUMAN. |
| Dilution | WB~~1:1000 ICC~~1:200 IP~~1:500 |
| Format | Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide, pH 7.3. |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Name | XRCC6 |
|---|---|
| Synonyms | G22P1 |
| Function | Single-stranded DNA-dependent ATP-dependent helicase that plays a key role in DNA non-homologous end joining (NHEJ) by recruiting DNA-PK to DNA (PubMed:11493912, PubMed:12145306, PubMed:20493174, PubMed:2466842, PubMed:7957065, PubMed:8621488, PubMed:9742108). Required for double-strand break repair and V(D)J recombination (PubMed:11493912, PubMed:12145306, PubMed:20493174, PubMed:2466842, PubMed:7957065, PubMed:8621488, PubMed:9742108). Also has a role in chromosome translocation (PubMed:11493912, PubMed:12145306, PubMed:20493174, PubMed:2466842, PubMed:7957065, PubMed:8621488, PubMed:9742108). Has a role in chromosome translocation (PubMed:11493912, PubMed:12145306, PubMed:20493174, PubMed:2466842, PubMed:7957065, PubMed:8621488, PubMed:9742108). The DNA helicase II complex binds preferentially to fork-like ends of double-stranded DNA in a cell cycle-dependent manner (PubMed:11493912, PubMed:12145306, PubMed:20493174, PubMed:2466842, PubMed:7957065, PubMed:8621488, PubMed:9742108). It works in the 3'-5' direction (PubMed:11493912, PubMed:12145306, PubMed:20493174, PubMed:2466842, PubMed:7957065, PubMed:8621488, PubMed:9742108). During NHEJ, the XRCC5-XRRC6 dimer performs the recognition step: it recognizes and binds to the broken ends of the DNA and protects them from further resection (PubMed:11493912, PubMed:12145306, PubMed:20493174, PubMed:2466842, PubMed:7957065, PubMed:8621488, PubMed:9742108). Binding to DNA may be mediated by XRCC6 (PubMed:11493912, PubMed:12145306, PubMed:20493174, PubMed:2466842, PubMed:7957065, PubMed:8621488, PubMed:9742108). The XRCC5-XRRC6 dimer acts as a regulatory subunit of the DNA-dependent protein kinase complex DNA-PK by increasing the affinity of the catalytic subunit PRKDC to DNA by 100-fold (PubMed:11493912, PubMed:12145306, PubMed:20493174, PubMed:2466842, PubMed:7957065, PubMed:8621488, PubMed:9742108). The XRCC5-XRRC6 dimer is probably involved in stabilizing broken DNA ends and bringing them together (PubMed:11493912, PubMed:12145306, PubMed:20493174, PubMed:2466842, PubMed:7957065, PubMed:8621488, PubMed:9742108). The assembly of the DNA-PK complex to DNA ends is required for the NHEJ ligation step (PubMed:11493912, PubMed:12145306, PubMed:20493174, PubMed:2466842, PubMed:7957065, PubMed:8621488, PubMed:9742108). Probably also acts as a 5'-deoxyribose-5-phosphate lyase (5'-dRP lyase), by catalyzing the beta-elimination of the 5' deoxyribose-5-phosphate at an abasic site near double-strand breaks (PubMed:20383123). 5'-dRP lyase activity allows to 'clean' the termini of abasic sites, a class of nucleotide damage commonly associated with strand breaks, before such broken ends can be joined (PubMed:20383123). The XRCC5-XRRC6 dimer together with APEX1 acts as a negative regulator of transcription (PubMed:8621488). In association with NAA15, the XRCC5-XRRC6 dimer binds to the osteocalcin promoter and activates osteocalcin expression (PubMed:12145306). Plays a role in the regulation of DNA virus-mediated innate immune response by assembling into the HDP-RNP complex, a complex that serves as a platform for IRF3 phosphorylation and subsequent innate immune response activation through the cGAS-STING pathway (PubMed:28712728). Negatively regulates apoptosis by interacting with BAX and sequestering it from the mitochondria (PubMed:15023334). Might have deubiquitination activity, acting on BAX (PubMed:18362350). |
| Cellular Location | Nucleus. Chromosome. Cytoplasm. Note=When trimethylated, localizes in the cytoplasm. |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
Single-stranded DNA-dependent ATP-dependent helicase. Has a role in chromosome translocation. The DNA helicase II complex binds preferentially to fork-like ends of double-stranded DNA in a cell cycle-dependent manner. It works in the 3'-5' direction. Binding to DNA may be mediated by XRCC6. Involved in DNA non-homologous end joining (NHEJ) required for double-strand break repair and V(D)J recombination. The XRCC5/6 dimer acts as regulatory subunit of the DNA-dependent protein kinase complex DNA-PK by increasing the affinity of the catalytic subunit PRKDC to DNA by 100-fold. The XRCC5/6 dimer is probably involved in stabilizing broken DNA ends and bringing them together. The assembly of the DNA-PK complex to DNA ends is required for the NHEJ ligation step. Required for osteocalcin gene expression. Probably also acts as a 5'-deoxyribose-5-phosphate lyase (5'-dRP lyase), by catalyzing the beta-elimination of the 5' deoxyribose- 5-phosphate at an abasic site near double-strand breaks. 5'-dRP lyase activity allows to 'clean' the termini of abasic sites, a class of nucleotide damage commonly associated with strand breaks, before such broken ends can be joined. The XRCC5/6 dimer together with APEX1 acts as a negative regulator of transcription.
References
Chan J.Y.,et al.J. Biol. Chem. 264:3651-3654(1989).
Reeves W.H.,et al.J. Biol. Chem. 264:5047-5052(1989).
Griffith A.J.,et al.Mol. Biol. Rep. 16:91-97(1992).
Ota T.,et al.Nat. Genet. 36:40-45(2004).
Halleck A.,et al.Submitted (JUN-2004) to the EMBL/GenBank/DDBJ databases.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.