ANGPTL3 Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

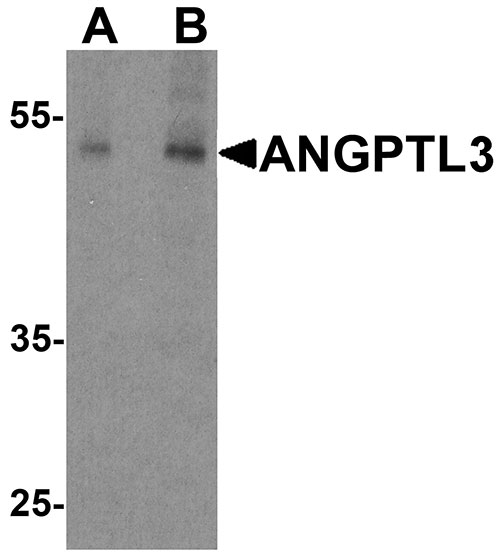
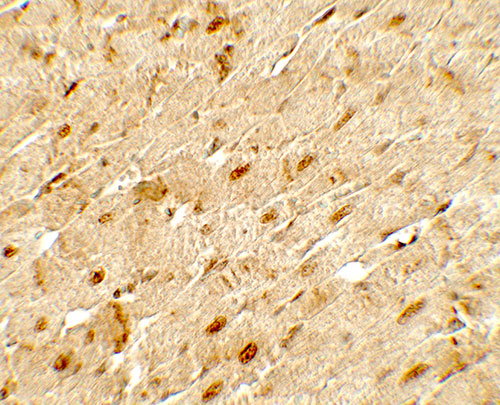
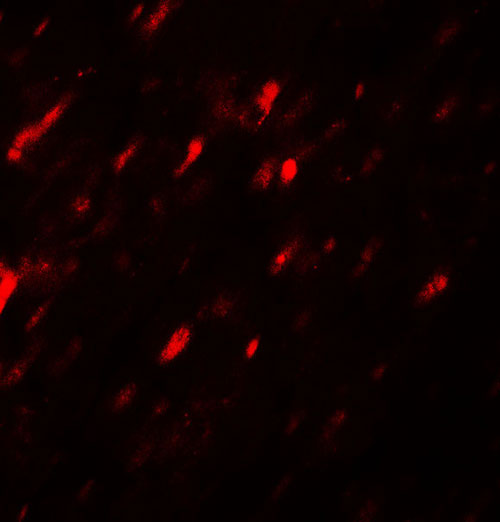
Application
| WB, IHC-P, IF, E |
|---|---|
| Primary Accession | Q9Y5C1 |
| Other Accession | NP_055310, 7656888 |
| Reactivity | Human, Mouse, Rat |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Calculated MW | Predicted: 51 kDa Observed: 53 kDa |
| Application Notes | ANGPTL3 antibody can be used for detection of ANGPTL3 by Western blot at 1 - 2 µg/ml. Antibody can also be used for Immunohistochemistry at 5 µg/mL. For Immunoflorescence start at 20 µg/mL. |
| Gene ID | 27329 |
|---|---|
| Target/Specificity | ANGPTL3; ANGPTL3 antibody is human, mouse and rat reactive. ANGPTL3 antibody is predicted not to cross-react with other ANGPTL family proteins. |
| Reconstitution & Storage | ANGPTL3 antibody can be stored at 4℃ for three months and -20℃, stable for up to one year. |
| Precautions | ANGPTL3 Antibody is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | ANGPTL3 |
|---|---|
| Synonyms | ANGPT5 |
| Function | Acts in part as a hepatokine that is involved in regulation of lipid and glucose metabolism (PubMed:11788823, PubMed:12909640, PubMed:23661675, PubMed:25495645). Proposed to play a role in the trafficking of energy substrates to either storage or oxidative tissues in response to food intake (By similarity). Has a stimulatory effect on plasma triglycerides (TG), which is achieved by suppressing plasma TG clearance via inhibition of LPL activity. The inhibition of LPL activity appears to be an indirect mechanism involving recruitment of proprotein convertases PCSK6 and FURIN to LPL leading to cleavage and dissociation of LPL from the cell surface; the function does not require ANGPTL3 proteolytic cleavage but seems to be mediated by the N- terminal domain, and is not inhibited by GPIHBP1 (PubMed:12097324, PubMed:19318355, PubMed:20581395). Can inhibit endothelial lipase, causing increased plasma levels of high density lipoprotein (HDL) cholesterol and phospholipids (PubMed:17110602, PubMed:19028676). Can bind to adipocytes to activate lipolysis, releasing free fatty acids and glycerol (PubMed:12565906). Suppresses LPL specifically in oxidative tissues which is required to route very low density lipoprotein (VLDL)-TG to white adipose tissue (WAT) for storage in response to food; the function may involve cooperation with circulating, liver-derived ANGPTL8 and ANGPTL4 expression in WAT (By similarity). Contributes to lower plasma levels of low density lipoprotein (LDL)-cholesterol by a mechanism that is independent of the canonical pathway implicating APOE and LDLR. May stimulate hypothalamic LPL activity (By similarity). |
| Cellular Location | Secreted {ECO:0000250, ECO:0000305|PubMed:11877390}. Cell projection, lamellipodium {ECO:0000250|UniProtKB:Q9R182}. Note=Colocalized with HSPG2 and activated ITGB3 on podocytes. {ECO:0000250|UniProtKB:Q9R182} |
| Tissue Location | Expressed principally in liver. Weakly expressed in kidney. Binds to adipocytes. Increased expression and colocalization with activated ITGB3 in glomeruli of patients with nephrotic syndrome showing effaced podocyte foot processes (at protein level) |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
The angiopoietin-related protein 3 (ANGPTL3) is a member of a family of secreted proteins that function in angiogenesis (1). ANGPTL3 is processed into an N-terminal coiled-coil domain-containing chain and a C-terminal fibrinogen chain. The N-terminal chain is important for lipid metablism, while the C-terminal chain may be involved in angiogenesis (2,3). Recent experiments have shown that a deficiency of ANGPTL3 is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids, suggesting that ANGPTL3 may also play a role in modulating glucose metabolism (4).
References
Conklin D, Gilbertson D, Taft DW, et al. Identification of a mammalian angiopoietin-related protein expressed specifically in liver. Genomics 1999; 62:477-82.
Koishi R, Ando Y, Ono M, et al. Angptl3 regulates lipid metabolism in mice. Nat. Genet. 2002; 30:151-7.
Camenisch G, Pisabarro MT, Sherman D, et al. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo.J. Biol. Chem. 2002; 277:17281-90.
Robciuc MR, Maranghi M, Lahikainen A, et al. Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler. Thromb. Vasc. Biol. 2013; 33:1706-13.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.