Anti-GLUTAMATE DEHYDROGENASE (Bovine Liver) (RABBIT) Antibody
Glutamate Dehydrogenase Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

| Host | Rabbit |
|---|---|
| Conjugate | Unconjugated |
| Target Species | Bovine |
| Reactivity | Bovine |
| Clonality | Polyclonal |
Application
| WB, E, IP, I, LCI |
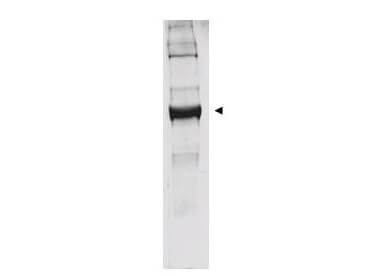
| Application Note | This antibody has been tested by western blot. Specific conditions for reactivity should be optimized by the end user. Bovine glutamate dehydrogenase exists as a homohexamer located within the mitochondrial matrix. Expect a band approximately 56 kDa in size corresponding to glutamate dehydrogenase monomer subunit by western blotting in the appropriate cell or tissue extract. Anti-Glutamate Dehydrogenase Antibody is suitable for use in ELISA. |
| Physical State | Lyophilized |
| Buffer | 0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2 |
| Immunogen | This antibody was prepared from whole rabbit serum produced by repeated immunizations with a full length Glutamate Dehydrogenase protein isolated from Bovine Liver. |
| Reconstitution Volume | 2.0 mL |
| Reconstitution Buffer | Restore with deionized water (or equivalent) |
| Preservative | 0.01% (w/v) Sodium Azide |
| Gene ID | 281785 |
|---|---|
| Other Names | 281785 |
| Purity | This product was prepared from monospecific antiserum by a delipidation and defibrination. Assay by immunoelectrophoresis resulted in a single precipitin arc against anti-rabbit serum, purified and partially purified Glutamate Dehydrogenase [Bovine Liver]. BLAST analysis was used to determine that cross reactivity is suggested for both mitochondrial and brain isoforms (GDH1 and GDH2), from both bovine and human sources. Additionally similar reactivity is suggested for most primate species including green monkey, white gibbon, chimpanzee orangutan, and gorilla. A high degree of sequence homology is also noted for GDH from chicken, mouse, rat, dog, and other mammals as well as Xenopus tropicalis, zebrafish, rainbow trout and Atlantic salmon. Cross reactivity against Glutamate Dehydrogenase from other tissues and species may occur but have not been specifically determined. |
| Storage Condition | Store vial at 4° C prior to restoration. For extended storage aliquot contents and freeze at -20° C or below. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. This product is stable for several weeks at 4° C as an undiluted liquid. Dilute only prior to immediate use. |
| Precautions Note | This product is for research use only and is not intended for therapeutic or diagnostic applications. |
| Name | GLUD1 |
|---|---|
| Synonyms | GLUD |
| Function | Mitochondrial glutamate dehydrogenase that converts L- glutamate into alpha-ketoglutarate. Plays a key role in glutamine anaplerosis by producing alpha-ketoglutarate, an important intermediate in the tricarboxylic acid cycle (PubMed:14659072, PubMed:4365183). Plays a role in insulin homeostasis (By similarity). May be involved in learning and memory reactions by increasing the turnover of the excitatory neurotransmitter glutamate (By similarity). |
| Cellular Location | Mitochondrion {ECO:0000250|UniProtKB:P00367}. Endoplasmic reticulum {ECO:0000250|UniProtKB:P00367}. Note=Mostly translocates into the mitochondria, only a small amount of the protein localizes to the endoplasmic reticulum. {ECO:0000250|UniProtKB:P00367} |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
Glutamate is a major excitatory neurotransmitter. One enzyme central to the metabolism of glutamate is glutamate dehydrogenase (GDH1; EC 1.4.1.3), that catalyzes the reversible deamination of L-glutamate to 2-oxoglutarate using NAD+ or NADP+. Mammalian GDH is composed of six identical subunits, and the regulation of GDH is very complex. It has been a major goal to identify the substrate and regulatory binding sites of GDH. It is only in recent years that the three-dimensional structure of GDH from microorganisms is available. Very recently, crystallization of bovine liver GDH was reported for the first time from the mammalian sources. However, remarkably little is known about the detailed structure of mammalian GDH, especially the brain enzymes.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.