Anti-Neuraminidase (Neu2) (RABBIT) Antibody
Neuraminidase Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

| Host | Rabbit |
|---|---|
| Conjugate | Unconjugated |
| Target Species | Human |
| Reactivity | Human |
| Clonality | Polyclonal |
Application
| WB, IHC, E, IP, I, LCI |
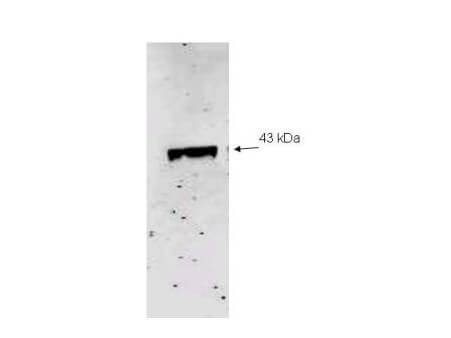
| Application Note | Anti-NEU2 Antibody is has been tested by western blot and ELISA and suitable for immunocytochemistry and immunoprecipitation, transfected cell culture, and primary cell culture. A single band of the expected apparent molecular weight (43 kDa) was observed at a 1:500 dilution incubated for 1 h at room temperature. A second lower molecular weight band may represent a truncated form of this protein. Neuraminidase is not very abundant in most tissues and its detection using this antibody may require further optimization. Researchers should determine optimal titers for other applications. |
| Physical State | Liquid (sterile filtered) |
| Buffer | 0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2 |
| Immunogen | This affinity purified antibody was prepared from whole rabbit serum produced by repeated immunizations with a synthetic peptide corresponding to an internal portion near aa 100-125 of Human Neu2. |
| Preservative | 0.01% (w/v) Sodium Azide |
| Gene ID | 4759 |
|---|---|
| Other Names | 4759 |
| Purity | This is an affinity purified antibody produced by immunoaffinity chromatography using the immunizing peptide after immobilization to a solid phase. This antibody reacts with human Neu2. Based on sequence we expect this antibody to react with neuraminidase from other sources, although specific reactivity has not been confirmed. Cross-reactivity against Neu1 has not yet been established. Neuraminidases are highly conserved in mammals and therefore cross reactivity is expected with mouse and rat Neu2. |
| Storage Condition | Store vial at -20° C prior to opening. Aliquot contents and freeze at -20° C or below for extended storage. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. This product is stable for several weeks at 4° C as an undiluted liquid. Dilute only prior to immediate use. |
| Precautions Note | This product is for research use only and is not intended for therapeutic or diagnostic applications. |
| Name | NEU2 |
|---|---|
| Function | Exo-alpha-sialidase that catalyzes the hydrolytic cleavage of the terminal sialic acid (N-acetylneuraminic acid, Neu5Ac) of a glycan moiety in the catabolism of glycolipids, glycoproteins and oligosacharides (PubMed:14613940, PubMed:22228546). Recognizes sialyl linkage positions of the glycan moiety as well as the supramolecular organization of the sialoglycoconjugate. Displays preference for alpha- (2->3)-sialylated GD1a and GT1B gangliosides over alpha-(2->8)- sialylated GD1b, in both monomeric forms and micelles. Hydrolyzes monomeric GM1 ganglioside, but has no activity toward the miscellar form (PubMed:14613940). Has lower sialidase activity for glycoproteins such as fetuin and TF/transferrin that carry a mixture of alpha-(2->3) and alpha-(2->6)-sialyl linkages. Cleaves milk oligosaccharide alpha- (2->3)-sialyllactose, but is inactive toward alpha-(2->6)-sialyllactose isomer. Has no activity toward colominic acid, a homomer of alpha- (2->8)-linked Neu5Ac residues (PubMed:14613940). |
| Cellular Location | Cytoplasm, cytosol. |
| Tissue Location | Expressed in skeletal muscle, fetal liver and embryonic carcinoma cell line NT2-D1. |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
Neuraminidases or sialidases are exoglycosidases that catalyze the cleavage of α-glycosidically linked terminal N-acetyl neuraminic acid from sialylated glycoconjugates. They are widely spread in nature, occurring in viruses, bacteria, fungi, protozoa, birds and mammals. Together, the neuraminidases form a family of hydrolases that share a conserved active site and similar sequence motifs. Three types of neuraminidase are found in mammals and are defined as lysosomal, plasma membrane and cytosolic on the basis of their biochemical properties and subcellular distribution. Lysosomal N-acetyl-α-neuraminidase (NEU1) has significant primary structure characteristics of other mammalian and microbial sialidases with similar substrate specificity. However, unlike other members of this family, lysosomal neuraminidase requires the carboxypeptidase protective protein/cathepsin A (PPCA) for intracellular transport and lysosomal activation. The enzyme is only catalytically active when it is bound to PPCA and is a component of a high molecular weight, multi-protein complex containing PPCA, ß-galactosidase and N-acetylgalactosamine-6-sulfate sulfatase. Using a hamster Sial3 probe, Monti et al. (1999) identified the gene encoding sialidase-2, which they designated NEU2, from a human genomic library. The 2 putative exons of NEU2 encode a deduced 380-amino acid protein with a calculated molecular mass of 42.23 kD. The NEU2 protein has significant homology with the mammalian, viral, and bacterial sialidases. It shares over 72% similarity with the hamster and rat cytosolic sialidases and over 42% similarity with human NEU1. NEU2 contains a potential N-linked glycosylation site, 2 aspartic acid block consensus sequences, and an N-terminal F/YRIP sequence motif which is part of the active site of other sialidase enzymes. Monti et al. hypothesized that NEU2 has a cytosolic localization because it does not contain a cleavage site, transmembrane domain, or targeting motifs.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.