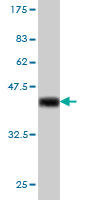
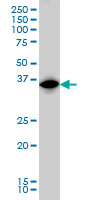
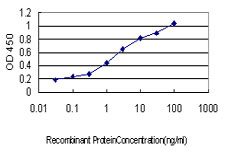
IRF1 Antibody (monoclonal) (M01)
Mouse monoclonal antibody raised against a partial recombinant IRF1.
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, E |
|---|---|
| Primary Accession | P10914 |
| Other Accession | NM_002198 |
| Reactivity | Human |
| Host | mouse |
| Clonality | Monoclonal |
| Isotype | IgG2a Kappa |
| Clone Names | 2E5 |
| Calculated MW | 36502 Da |
| Gene ID | 3659 |
|---|---|
| Other Names | Interferon regulatory factor 1, IRF-1, IRF1 |
| Target/Specificity | IRF1 (NP_002189, 216 a.a. ~ 325 a.a) partial recombinant protein with GST tag. MW of the GST tag alone is 26 KDa. |
| Dilution | WB~~1:500~1000 E~~N/A |
| Format | Clear, colorless solution in phosphate buffered saline, pH 7.2 . |
| Storage | Store at -20°C or lower. Aliquot to avoid repeated freezing and thawing. |
| Precautions | IRF1 Antibody (monoclonal) (M01) is for research use only and not for use in diagnostic or therapeutic procedures. |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
IRF1 encodes interferon regulatory factor 1, a member of the interferon regulatory transcription factor (IRF) family. IRF1 serves as an activator of interferons alpha and beta transcription, and in mouse it has been shown to be required for double-stranded RNA induction of these genes. IRF1 also functions as a transcription activator of genes induced by interferons alpha, beta, and gamma. Further, IRF1 has been shown to play roles in regulating apoptosis and tumor-suppressoion.
References
1.Defining critical roles for NF-?eB p65 and type I interferon in innate immunity to rhinovirus.Bartlett NW, Slater L, Glanville N, Haas JJ, Caramori G, Casolari P, Clarke DL, Message SD, Aniscenko J, Kebadze T, Zhu J, Mallia P, Mizgerd JP, Belvisi M, Papi A, Kotenko SV, Johnston SL, Edwards MR.EMBO Mol Med. 2012 Dec;4(12):1244-60. doi: 10.1002/emmm.201201650. Epub 2012 Nov 14.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.