IRE1p Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

Application
| WB, IF, ICC, E |
|---|---|
| Primary Accession | O75460 |
| Other Accession | O75460, 193806335 |
| Reactivity | Human, Mouse, Rat |
| Host | Rabbit |
| Clonality | Polyclonal |
| Isotype | IgG |
| Calculated MW | 109735 Da |
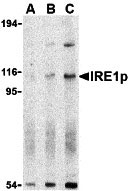
| Application Notes | IRE1p antibody can be used for the detection of IRE1p by Western blot at 0.5 - 2 µg/mL. Antibody can also be used for immunocytochemistry starting at 1 µg/mL. For immunofluorescence start at 10 µg/mL. |
| Gene ID | 2081 |
|---|---|
| Other Names | IRE1p Antibody: IRE1, IRE1P, IRE1a, hIRE1p, IRE1, Endoplasmic reticulum-to-nucleus signaling 1, endoplasmic reticulum to nucleus signaling 1 |
| Target/Specificity | ERN1; |
| Reconstitution & Storage | IRE1p antibody can be stored at 4℃ for three months and -20℃, stable for up to one year. As with all antibodies care should be taken to avoid repeated freeze thaw cycles. Antibodies should not be exposed to prolonged high temperatures. |
| Precautions | IRE1p Antibody is for research use only and not for use in diagnostic or therapeutic procedures. |
| Name | ERN1 (HGNC:3449) |
|---|---|
| Function | Serine/threonine-protein kinase and endoribonuclease that acts as a key sensor for the endoplasmic reticulum unfolded protein response (UPR) (PubMed:11175748, PubMed:11779464, PubMed:12637535, PubMed:19328063, PubMed:21317875, PubMed:28128204, PubMed:30118681, PubMed:36739529, PubMed:9637683). In unstressed cells, the endoplasmic reticulum luminal domain is maintained in its inactive monomeric state by binding to the endoplasmic reticulum chaperone HSPA5/BiP (PubMed:21317875). Accumulation of misfolded proteins in the endoplasmic reticulum causes release of HSPA5/BiP, allowing the luminal domain to homodimerize, promoting autophosphorylation of the kinase domain and subsequent activation of the endoribonuclease activity (PubMed:21317875). The endoribonuclease activity is specific for XBP1 mRNA and excises 26 nucleotides from XBP1 mRNA (PubMed:11779464, PubMed:21317875, PubMed:24508390). The resulting spliced transcript of XBP1 encodes a transcriptional activator protein that up-regulates expression of UPR target genes (PubMed:11779464, PubMed:21317875, PubMed:24508390). Acts as an upstream signal for ER stress-induced GORASP2-mediated unconventional (ER/Golgi-independent) trafficking of CFTR to cell membrane by modulating the expression and localization of SEC16A (PubMed:21884936, PubMed:28067262). |
| Cellular Location | Endoplasmic reticulum membrane; Single-pass type I membrane protein |
| Tissue Location | Ubiquitously expressed. High levels observed in pancreatic tissue. |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
IRE1p Antibody: Accumulation of malfolded proteins in the endoplasmic reticulum (ER) activates the unfolded protein response (UPR) and the upregulation of the ER molecular chaperones GRP78 and GRP 94. These proteins are normally bound to ER transmembrane proteins such as IRE1p and ATF6 but ER stress causes their dissociation. This allows IRE1p, a serine-threonine protein kinase to transduce the unfolded protein signal from the ER to the nucleus. IRE1p also has an endoribonuclease activity that is required to splice X-box binding protein (XBP1) mRNA converting it to a potent UPR transcriptional activation. Depletion of IRE1p through the expression of a dominant negative form of IRE1p has no effect on transfected cells, but cell death via apoptosis occurs under stress conditions that cause unfolded proteins to accumulate in the ER. Two alternatively spliced transcript variants encoding different isoforms have been found for this gene.
References
Little E, Ramakrishnan M, Roy B, et al. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit. Rev. Eukaryot. Gene Expr.1994; 4:1-18.
Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods2005; 35:373-81.
Bertolotti A, Zhang Y, Hendershot LM, et al. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol.2000; 2:326-32.
Shen J, Chen X, Hendershot L, et al. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell2002; 3:99-111.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.