Anti-AHA1 (RABBIT) Antibody
AHA1 Antibody
- SPECIFICATION
- CITATIONS
- PROTOCOLS
- BACKGROUND

| Host | Rabbit |
|---|---|
| Conjugate | Unconjugated |
| Target Species | Human |
| Reactivity | Human, Monkey |
| Clonality | Polyclonal |
Application
| WB, IHC, E, I, LCI |
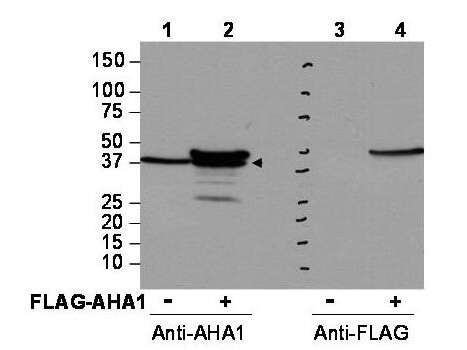
| Application Note | This affinity purified antibody has been tested for use in ELISA and western blotting. Specific conditions for reactivity should be optimized by the end user. Expect a band approximately 38-40 kDa in size corresponding to AHA1 protein by western blotting in the appropriate cell lysate or extract. |
| Physical State | Liquid (sterile filtered) |
| Buffer | 0.02 M Potassium Phosphate, 0.15 M Sodium Chloride, pH 7.2 |
| Immunogen | This affinity purified antibody was prepared from whole rabbit serum produced by repeated immunizations with a synthetic peptide corresponding to an internal region of human AHA1 protein. |
| Preservative | 0.01% (w/v) Sodium Azide |
| Gene ID | 10598 |
|---|---|
| Other Names | 10598 |
| Purity | This affinity purified antibody is directed against human AHA1 protein. The product was affinity purified from monospecific antiserum by immunoaffinity chromatography. A BLAST analysis was used to suggest cross-reactivity with AHA1 protein from human and chimpanzee based on 100% homology with the immunizing sequence. Reactivity against homologues from other sources is not known. |
| Storage Condition | Store vial at -20° C prior to opening. Aliquot contents and freeze at -20° C or below for extended storage. Avoid cycles of freezing and thawing. Centrifuge product if not completely clear after standing at room temperature. This product is stable for several weeks at 4° C as an undiluted liquid. Dilute only prior to immediate use. |
| Precautions Note | This product is for research use only and is not intended for therapeutic or diagnostic applications. |
| Name | AHSA1 |
|---|---|
| Synonyms | C14orf3 |
| Function | Acts as a co-chaperone of HSP90AA1 (PubMed:29127155). Activates the ATPase activity of HSP90AA1 leading to increase in its chaperone activity (PubMed:29127155). Competes with the inhibitory co- chaperone FNIP1 for binding to HSP90AA1, thereby providing a reciprocal regulatory mechanism for chaperoning of client proteins (PubMed:27353360). Competes with the inhibitory co-chaperone TSC1 for binding to HSP90AA1, thereby providing a reciprocal regulatory mechanism for chaperoning of client proteins (PubMed:29127155). |
| Cellular Location | Cytoplasm, cytosol. Endoplasmic reticulum. Note=May transiently interact with the endoplasmic reticulum |
| Tissue Location | Expressed in numerous tissues, including brain, heart, skeletal muscle and kidney and, at lower levels, liver and placenta. |

Thousands of laboratories across the world have published research that depended on the performance of antibodies from Abcepta to advance their research. Check out links to articles that cite our products in major peer-reviewed journals, organized by research category.
info@abcepta.com, and receive a free "I Love Antibodies" mug.
Provided below are standard protocols that you may find useful for product applications.
Background
This antibody is designed, produced, and validated as part of a collaboration between Rockland and the National Cancer Institute (NCI) and is suitable for Cancer, Immunology and Nuclear Signaling research. Activator of Hsp90 ATPase (AHA1) stimulates the inherent ATPase cycle of Hsp90, which is essential for its chaperone activity in vivo. The activation and/or stability of many of the key regulatory and signaling proteins of the eukaryotic cell depend on their interaction with the Hsp90 molecular chaperone. Hsp90 is assisted and regulated by co-chaperones that participate in an ordered series both to assist client-protein recruitment or release and to modulate progress through the ATPase coupled chaperone cycle. Structural analysis and mutagenesis show that binding of the N-terminal domain of AHA1 to Hsp90 promotes a conformational switch in the middle-segment catalytic loop (aa 370–390) of Hsp90 that exposes the catalytic Arg380 and enables its interaction with ATP in the N-terminal nucleotide-binding domain of the chaperone. Recent studies show that AHA1 modulates Hsp90-dependent stability of the folding of the cystic fibrosis transmembrane conductance regulator (CFTR) in the endoplasmic reticulum (ER). Down-regulation of AHA1 rescues misfolding of CFTR in cystic fibrosis.
If you have used an Abcepta product and would like to share how it has performed, please click on the "Submit Review" button and provide the requested information. Our staff will examine and post your review and contact you if needed.
If you have any additional inquiries please email technical services at tech@abcepta.com.













 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.