Activated monocyte-derived macrophages (MDM) contribute to the COVID-19 cytokine storm by releasing massive amounts of pro-inflammatory cytokines. CCL , CC- chemokine ligand; CXCL10, CXC- chemokine ligand 10; ISG, interferon- stimulated
gene; ITAM, immunoreceptor tyrosine- based activation motif; TRAM, TRIF- related adaptor molecule. Monocytes differentiate into pro-inflammatory macrophages though activation of Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathways. Activated natural killer (NK) cells and T cells further promote the recruitment and activation of monocyte-derived macrophages through the production of granulocyte–macrophage colony- stimulating factor (GM- CSF), tumor necrosis factor (TNF) and interferon-γ (IFNγ). Oxidized phospholipids (OxPLs) accumulate in infected lungs and activate MDMs through the Toll- like receptor 4 (TLR4)–TRAF6–NF-κB pathway. Virus sensing can trigger TLR7 activation through viral single-stranded RNA recognition. Type-I INF might induce the expression of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) entry receptors (ACE2, CD147), enabling the virus to access the macrophage cytoplasm and activate the NLRP3 inflammasome, leading to the secretion of IL-1β and/or IL-18. The engagement of Fcγ receptors (FcγRs) by anti-spike protein IgG immune complexes can contribute to increased inflammatory activation of MDMs.
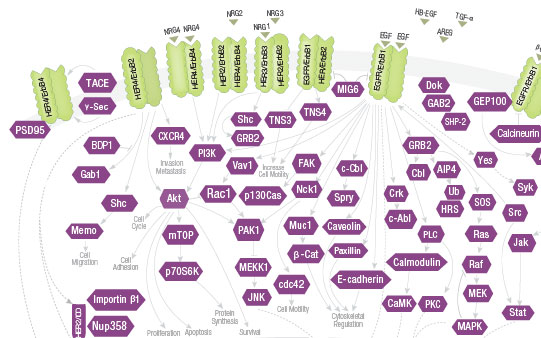
Her/ErbB receptor tyrosine kinases are activated upon
ligand binding and dimerization. They signal through
Akt, MAPK, and other pathways to regulate proliferation,
differentiation, apoptosis, migration, and motility.
Reduced ErbB signaling is linked to neurodegenerative
diseases such as multiple sclerosis and Alzheimer's.
Cancers associated with mutation or increased
expression of Her/ErbBs include lung, breast, stomach, colorectal, head and neck, and pancreatic cancers.
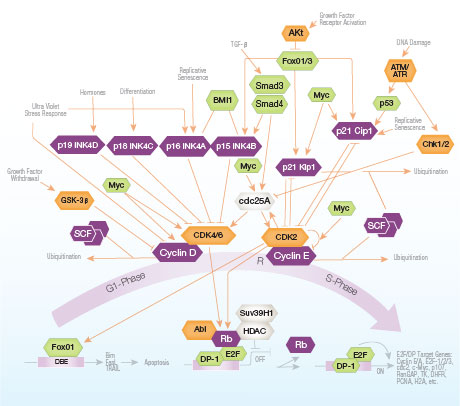
Cell cycle arrest provides time crucial for repair of DNA damage, preserving
genomic integrity. Growth arrest activates through checkpoint pathways that
delay cell cycle progression. The balance between cell differentiation and proliferation
is regulated transcriptionally; in the cell cycle the G1 to S-phase transition is the primary
control point. Only prior to this can cells be directed to differentiation, otherwise they progress
to the proliferative cycle autonomously.
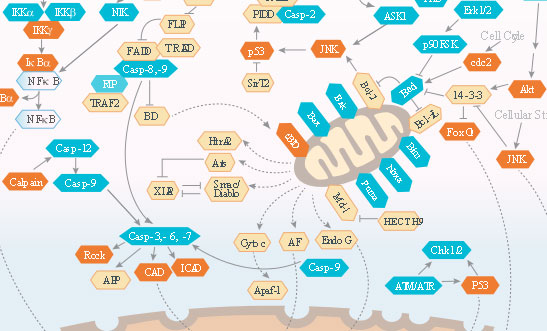
Apoptosis (programmed cell death) is characterized by cell shrinkage, membrane blebbing, phagocytic engulfment of the fragmented cell, DNA fragmentation and mitochondrial release of cytochrome C. Tightly regulated termination is essential to cull unneeded, aging, mutated, or infected cells. Dysregulation of death/survival signals in the apoptotic pathway is implicated in a broad range of human diseases. Controlling fidelity of the apoptotic pathways may lead to new methods to treat challenging diseases.
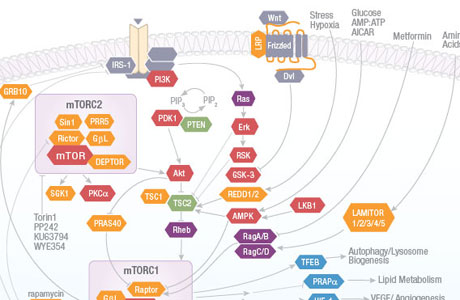
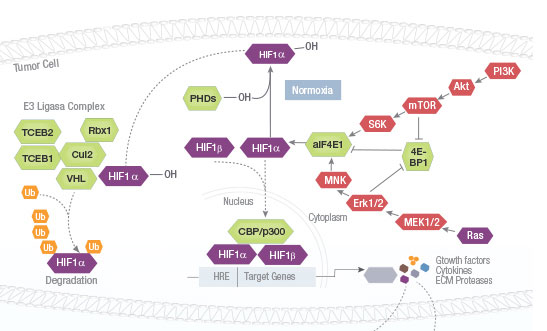
The mammalian target of rapamycin (mTOR) signaling pathway integrates intracellular and extracellular signals to regulate cell metabolism, growth, proliferation and survival. This pathway is activated during a range of cellular processes (tumor formation, angiogenesis, insulin resistance, adipogenesis, T-lymphocyte activation, etc.) and is dysregulated in diseases such as cancer and diabetes. mTOR inhibitors such as rapamycin and its analogues are used in treatment of solid tumors, organ transplantation, circulatory disease and rheumatoid arthritis.
Angiogenesis is the process of creating new blood vessels from preexisting blood vessels. This process is essential for healing, growth, development, maintenance, and wound repair in an organism. The angiogenesis pathway is controlled by balancing stimulatory and inhibitory factors. When this balance is disrupted, abnormal blood vessel growth occurs. Overgrowth or lack of growth may be an underlying contributor to medical conditions including cancer, skin diseases, age-related blindness, diabetic wounds that do not heal, heart disease, and strokes.
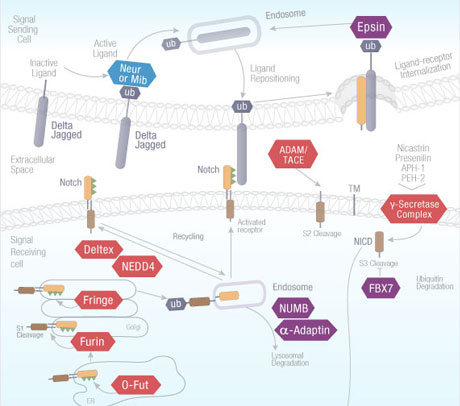
The Notch signaling pathway is an evolutionarily conserved pathway operating in multicellular organisms. This pathway plays a significant role in cell differentiation during embryonic development and maintenance of adult tissue homeostasis. Notch receptors are single-pass transmembrane proteins that bind in calcium-dependent, non-covalent interactions with Notch proteins to activate signaling. This pathway is of enduring interest to pharmaceutical researchers due to its regulatory effects on neurological, cell growth, and cardiovascular systems related to human diseases. Notch signaling promotes cellular proliferation during neurogenesis, and is inhibited by the protein Numb to promote neural differentiation. Key mutations in Notch receptors leader to deposition of proteins common in leukemias and lymphomas, and both Notch receptor and ligand mutations have been tied to disorders including Aagille syndrome and pathologies of the cerebral arteries.
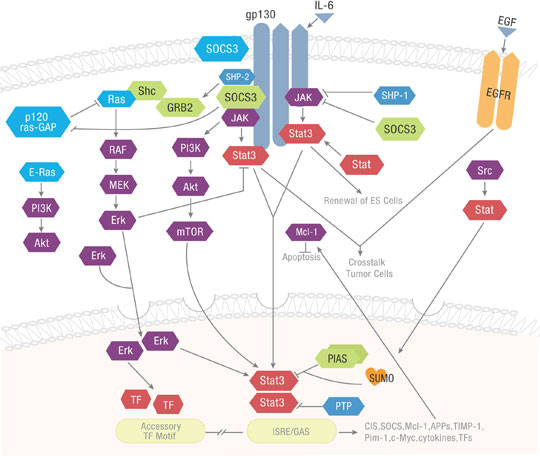
The Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway cascades to transduce cellular signals for development and homeostasis. The JAK-STAT signaling pathway is a critical component of cytokine receptor and growth factor systems regulating growth, survival, differentiation, and activation of the immune system. JAK activation plays a role in specific cellular processes such as cell proliferation, cell migration and apoptosis. Mutational downregulation of the JAK/STAT pathway affects hematopoiesis, immune system development, adipogenesis, and other processes. Upregulation caused by specific mutations contribute to inflammatory disease, erythrocytosis, and cancers, among other disorders.
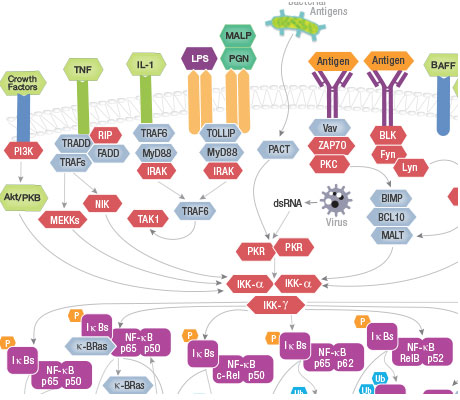
Nuclear factor-kB (NF-kB)/Rel proteins control genes involved in inflammation, innate and adaptive immunity, B-cell development inflammation, and stress responses.
In the canonical NF-kB pathway, IkB proteins bind and inhibit NF-kB/Rel proteins. A host of receptors activate an IKK complex that phosphorylates IkB proteins, triggering ubiquitination and proteasomal degradation, liberating NF-kB/Rel complexes which ultimately migrate to the nucleus to upregulate target gene expression.
In the noncanonical pathway, specific receptor signaling activates kinase NIK, which activates IKKa complexes that phosphorylate residues in NF-kB2 p100. This process promotes ubiquitination and proteasomal cleavage to NF-kB2 p52. NF-kB p52/RelB complexes move to the nucleus to induce target gene expression.
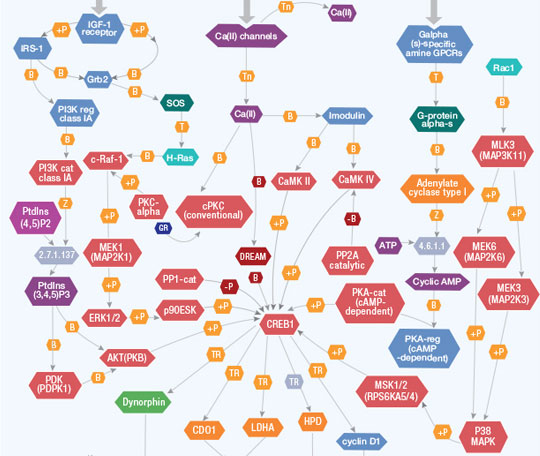
Extracellular stimuli summon changes in gene expression in target cells by activating intracellular protein kinase cascades that phosphorylate nuclear transcription factors. Transcription factor CREB1 (cyclic AMP (cAMP) responsive element binding protein 1) activates transcription of the target genes in response to a wide array of stimuli, including peptide hormones, growth factors, and neuronal activity. CREB1 is essential to a variety of cellular processes, including proliferation, differentiation, and adaptive responses. CREB proteins are critical for learning and memory and contribute to neuronal adaptation to drugs of abuse and hormonal control of metabolic processes, including regulation of gluconeogenesis by hormones glucagon and insulin.














 Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them.
Foundational characteristics of cancer include proliferation, angiogenesis, migration, evasion of apoptosis, and cellular immortality. Find key markers for these cellular processes and antibodies to detect them. The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
The SUMOplot™ Analysis Program predicts and scores sumoylation sites in your protein. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle. The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.
The Autophagy Receptor Motif Plotter predicts and scores autophagy receptor binding sites in your protein. Identifying proteins connected to this pathway is critical to understanding the role of autophagy in physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer.